Barbour’s Map Turtle (Graptemys barbouri)

Photos by J.D. Willson unless otherwise noted
| Description: The riverine Barbour’s map turtle displays a high degree of sexual dimorphism. Typically, males have a carapace length of less than 12 cm (4.7 in) while females may reach 33 cm (12.9 in) long. Females also have a much larger head than males of the same species. In addition, females darken markedly once they reach maturity, retaining few signs of the patterning of juveniles and males. The color of the carapace is greenish or olive brown, though adults found in the wild will usually be covered in algae. Skin coloration of the head, legs, and tail ranges from dark olive to nearly black with faint yellow or yellowish green markings. The plastron is yellow, although the transverse seams are lined with dark pigmentation. Barbour’s map turtles have keeled carapaces that contain black-tipped spines or knobs. Both the pleural scutes and upper surfaces of the marginal scutes have yellow, semi-circular markings that resemble the letter C. The lower parts of the marginal scutes have dark markings on a light background. A defining characteristic of this species is an interorbital blotch on top of the head. This blotch ends in a point above the nose and is connected to blotches or crescents immediately behind each eye. A light transverse or curved bar can be found on the underside of the chin.
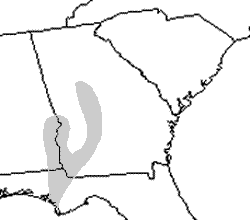
Distribution and Habitat: Barbour’s map turtles can be found in the Gulf Coastal Plain in the Apalachicola and Choctawhatchee River systems. This limited range includes parts of southeast Alabama, southwest Georgia, and the Florida Panhandle. The Chattahoochee, Flint, and Chipola Rivers in which these turtles reside are clear flowing with limestone rock and cobble bottoms. They are also rich in mollusks and contain many fallen trees and exposed rocks for basking. Reproduction and Development: The nesting season for Barbour’s map turtles is from June to August. The only reports of mating have been during the winter months from captive turtles. Nests are laid on sandbars or riverbanks as far away as 200 m (656 ft) from the water. They typically contain 4-11 eggs that hatch in late August and September. The temperature during incubation determines the sex ratio of hatchlings as with many turtle species; warmer temperatures result in a higher number of females. Males become sexually mature at 4 years, while females reach maturity at 15-20 years. Habits: Basking is a common occurrence in this species of map turtle and usually takes place on rocks, tree branches, or tree stumps at a safe distance from shore. They have been recorded to bask at temperatures as low as 10°C, but in general there is not much activity in colder months. Wary Barbour’s map turtles will dive into the water at the slightest disturbance. Due to their powerful jaws, females maintain a diet of primarily aquatic snails and freshwater mussels. Females have also been known to scrape freshwater sponges off hard substratum. Males and juveniles tend to ingest softer-bodied invertebrates such as caddisflies and dragonfly nymphs. Raccoons and other mammals cause the most harm to eggs and hatchlings, but introduced fire ants have also become a major problem. Fire ants are likely to pose a problem for many egg-laying reptiles, but we have few data quantifying those impacts. Conservation: The Barbour’s map turtle is listed as a vulnerable species by the IUCN. A number of conservation issues have contributed to their decline. First, free-flowing rivers that comprise the natural habitat of these turtles are slowly but surely being replaced by deep reservoirs with silt bottoms. Siltation destroys native mussel populations. Non-native Asian clams have invaded some streams within the Barbour’s map turtle’s range, providing a replacement food source. Various cases of unexplained shell rot is likely due to river pollution, and collection for the pet trade has also impacted populations of map turtles. Invasive plants have taken their toll on suitable nesting locations by colonizing sandbars, especially those that no longer flood. Pertinent References: Conant, Roger, and Joseph T. Collins. A Field Guide to Reptiles & Amphibians: Eastern and Central North America. 3rd ed. Boston: Houghton Mifflin, 1998. Jensen, John B., Carlos D. Camp, Whit Gibbons, and Matt J. Elliott. Amphibians and Reptiles of Georgia. Athens: University of Georgia, 2008. Account Author: Lindsay Partymiller |
